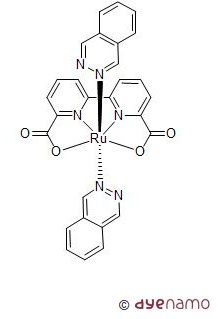
 The use of solar light to induce water splitting into its components, hydrogen and oxygen, has attracted an increasing interest. Interestingly enough, the most challenging step in light-induced water splitting is identifying good catalysts for the oxidation, formally offering the less interesting oxygen gas as a product. Nevertheless, this is highly necessary in a light-driven device. Thus, the interest for man-made molecular catalysts for oxidation of water has become a very hot and central research topic due to the urgent needs of sustainable solar energy systems as well as green chemical processes. In both cases, water has been considered as the ultimate source of proton, electron and oxygen without the consumption of classical chemical reagents which cause severe environmental issues. Although nature adopted Mn based catalyst for water oxidation in Photosystem II, man-made water oxidation catalysts based on molecular Mn complexes have so far received very limited success. The breakthrough for molecular water oxidation catalysts, however, took place when using other transition metal complexes, for example Ru and Ir (L. Sun et al., Nature Chem. 2012, 4, 418-423). Such systems can be chemically driven, electrochemically driven and even light-driven. By using similar concept as in dye-sensitized solar cells and in combination with the water oxidation catalyst on the photoactive anode, dye sensitized photo-electrochemical cells can also be made for light-driven water splitting.
Dyenamo is offering a set of components for solar fuels as standard materials. Other materials can be obtained on demand.
For more information on solar fuels, we refer to the publication "Highly efficient and robust molecular ruthenium catalysts for water oxidation" (L. Duan, C. M. Araujo, M. S. G. Ahlquist, L. Sun, PNAS 2012, DOI:10.1073/pnas.1118347109).
|